Thursday, February 2, 2023
Retrofit L2

In June 2021, we started to work on a project we named “Retrofit L2”. The assignment for this project was a big challenge, because in this project, we had to solve audit findings and compliance with regulations (including the new annex 1) and significantly reduce API losses during production and, last but not least, prepare Line 2 for a central formulation area. At the same time, the retrofitted line had to be able to produce both aqueous and non-aqueous products (organic solvents). For one of our products, it had to be able to carry out the flushing process (i.e. wash filters before production) in a sterile way without any risk for the product. The retrofit of L2 meant to replace the product pipeline of Line 2 with a mobile/portable solution for sterile filtration and change the concept of sterile filtration (redundancy) at the same time. The solution consists of three main parts, a mobile vessel, a filtration skid and a TCU (Temperature Control Unit). Since we had to replace the aseptic part, i.e. a very risky part of the production equipment, we decided that we had to preserve the functionality of the original product pipeline.
The project started with gathering information and discussions about possible solutions for the sterile filtration. After several workshops, we agreed on a basic principle, drew a basic diagram and described our requirements in a URS (User Requirement Specification). After many negotiations, we finally agreed with IPROS, a Slovenian company, and they became our main partner.
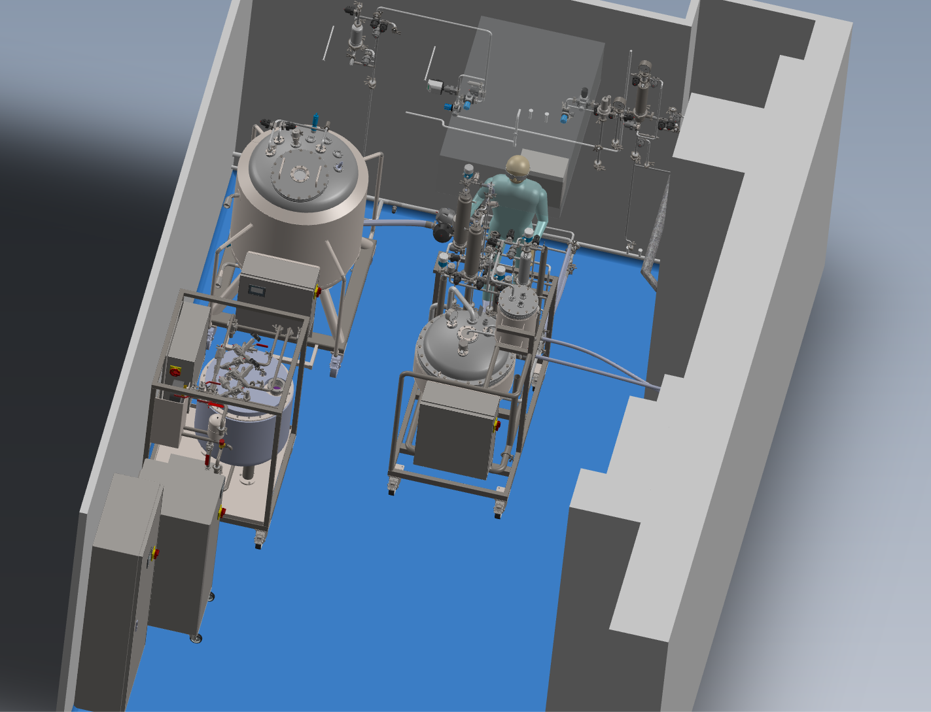
Step by step, we created the basic design, then the detail design, complete drawings and specifications of all components. The IPROS company then started to manufacture the equipment. After completion, we successfully tested the equipment as part of FAT (Factory Acceptance Test) in April 2022. In the meantime, we had to prepare Line 2 for the installation, which meant that we had to modify the premises, install a laminar field and create connection points. We also had to resolve the layout of the equipment and ensure easy operation of the equipment, because all the equipment altogether take up a lot of space. Therefore, we created a 3D model to simulate the layout and to decide where to place everything.
After the installation of the equipment during spring shutdown in April 2022, the original pipeline, which was impacted by the connection of the new equipment, was qualified. The qualification included a media fill using the original section. This fulfilled the requirement for a valid condition of the original pipeline and we were able to start commercial production.
As we were producing commercial batches using the original pipeline, we started to work on automation and qualification of the Retrofit. We divided this phase into several stages, the first stage concerned the automation stages for CIP and SIP, after which we could complete an SAT (Site Acceptance Test) and take over the equipment from IPROS. After taking over the equipment, we continued programming the automation phases and also with the qualifications.
The first qualification focused on CIP cleaning processes and SIP sterilization processes. The qualification was carried out by EDR, an external company, and included drying phases. This was the first prerequisite for the production of media fill using the new equipment.
The media fill contained three consecutive batches and included the validation of new processes and determination of new holding periods. All three runs, including samples, were satisfactory.
From September to December 2022, we focused on active programming and especially testing which was not easy with the production still running. The tests could only be performed outside of production, so we sacrificed many weekends. Part of the testing included the creation of procedures and operation cards.
Finally, we reached the functional stages and were able to start first test production of a product and then validation. The project is currently at the stage of completion and handover for production. For a more detailed overview + photo see below.
In total, the project took about a year and a half, during the project we produced about 150 documents, programmed and tested 44 automation phases. The result of the project is a functional mobile solution for sterile filtration with the possibility to produce both aqueous and non-aqueous products with high assurance of product sterility and very low API losses.